A New Rule For Biotech Investing: Chase Reality Not Dreams
Set for Phase 2 trials,[1] THAR’s breakthrough targets an incurable problem and could put the startup on the fast track
Two biotech veterans have transformed a mundane technology into a
breakthrough that could stall, or even reverse, the effects of an incurable
liver disease.[2]
Their novel breakthrough could offer hope of lasting relief to millions
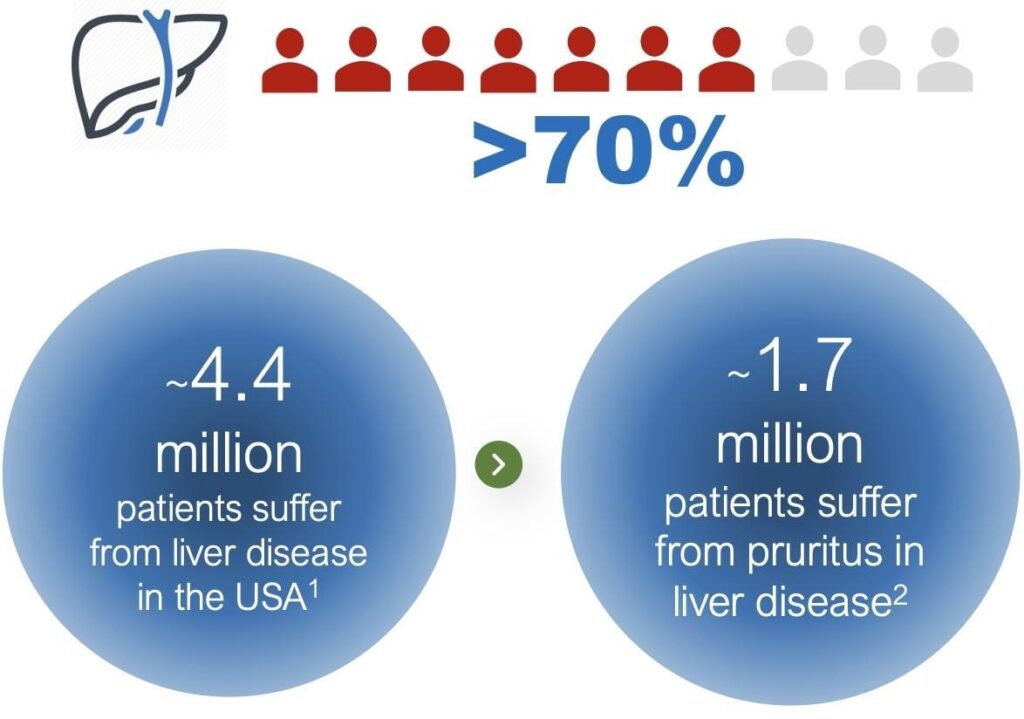
of people who suffer the brutal side-effect of a liver disease called Primary Biliary Cholangitis.[3]
The side effect, Chronic Pruritis, causes raging and unrelenting itching in more than 70 percent of those with PBC disease.[4]
Researchers have long lamented the unmet needs of pruritis sufferers whose itching can become so painful that it drives them to suicide.[5]
That could make this the perfect moment to consider snapping up shares in
Tharimmune Inc. (Nasdaq: THAR).
That’s because Tharimmune’s novel drug TH104, aimed at easing moderate to
severe Chronic Pruritis, sailed through Phase 1 and is now ready for Phase
2 clinical trials.[6]
Of course, trials can’t be rushed.
Still, Tharimmune Inc. (Nasdaq: THAR)
estimates that a $350-million Chronic Pruritis market awaits TH104.[7]
The total Chronic Pruritis market from other causes, such as atopic dermatitis, could be as great as $15 billion.[8]
That’s a lot of pent-up demand.
So, eager eyes are on the Phase 2.
Each Step Could Potentially Maximize Profits
In theory, each step along the clinical-trials path could de-risk the speculation on a startup biotech. Though it looks set to start Phase 2 trials, there are three other factors that could de-risk Tharimmune (Nasdaq: THAR).
The first is that the active drug in TH104 is delivered via a new form of transdermal patch. Called transmucosal, because the super thin film attaches to the inside of the cheek that’s unlike thicker nicotine or hormone patches that are placed on the skin.[9]
What makes this delivery system even more novel is that the patch’s drug is quickly released directly into the bloodstream instead of having to be digested in the gut or the liver, which can reduce a drug’s concentration in the blood. The benefit is known as avoiding First Pass Liver Metabolism.[10]
Another unique factor that could lessen investors’ risk is that the active drug in TH104, Nalmefene, has already met FDA approval, but only for treating opioid overdoses.[11] Tharimmune’s clinical trials are designed to make sure the drug is safe for other uses, such as Chronic Pruritis.
Its management and leadership are the third factor that would suggest a reduced-speculation risk for Tharimmune (Nasdaq: THAR).
CEO Randy Milby was CorMedix’s Chief Executive Officer. He led the publicly traded biotech from a $3 million market cap to a peak of $350 million. He led the effort to get Cormedix’s antimicrobial Neutrolin approved in the EU. Milby also knows both sides of the biotech business from his days at Goldman Sachs and Dupont Merck.[12]
COO Sireesh Appajosyula was senior vice president of Corporate Development and Operations of 9 Meters Biopharma, Inc. (Nasdaq: NMTR). Appajosyula also spent eight years at Salix Pharmaceuticals (Nasdaq: SLXP) in various roles in medical affairs, product commercialization and business development, as well as various roles at Amgen Inc., Critical Therapeutics, Inc. and Sanofi.[13]
An Ideal Formula For Success Is A Drug To Treat An Orphan Disease[14]
That Mibly and Appajosyula have transformed the fast-acting drug into a
quick dissolving patch is great news for any one of the 1.7 million people
who suffer from Chronic Pruritus related to chronic liver diseases in the U.S.
alone.
But market size is not the only positive report coming from the fledgling company.
That it’s set for Phase 2 human trials could put Tharimmune (Nasdaq: THAR) lightyears ahead of biotech companies attempting to create an entirely new drug.
And if Tharimmune stays on its current timeline, it could be ready to release its cure to the world by the end of 2025![15b]
That means that in a little over a year and half Tharimmune (Nasdaq: THAR) could have a viable product ready for market… not just any market either… rather it will be an exclusive market.
That’s because patent laws combined with FDA testing requirements means there arguably won’t be a single copycat competitor for at least a decade after that.[16]
The Future Of Biotech Is Here
It all means that what Tharimmune (Nasdaq: THAR) could be doing with TH104 is creating a brand-new market where none had been before.
Biotech investors crave gang-busting, market-disrupting drugs. This could be better than those.
Because it can’t be stressed enough, a single drug that creates a new market also means it could be years ahead of competitors.
That means that right now the only way a competitor can get its hands on TH104 is to buy a license from Tharimmune or buy the company.
Yes, a buyout or merger is a wildcard, but a $15 billion market suggests Tharimmune (Nasdaq: THAR) could be worth a fortune.
Biotech Investing Means Diversification…
But That Means A Mix Of Drugs At Differing Stages…
Tharimmune’s TH104, Set For Phase 2, Fits Into That Mix
Early-stage biotechs, by their nature, are the province of aggressive investors.
Knowing your risk profile is paramount.
Of course, you should never risk more than you are comfortable losing.
And understand the inherent risks that come with investing in a new company.
Smart early-stage biotech investors often diversify through exposure to companies in a number of different stages of development.
As its TH104 heads into Phase 2 trials, and is led by top management, Tharimmune (Nasdaq: THAR) has the look of a potential winner.
There’s a link below that leads to a comprehensive look at Tharimmune
(Nasdaq: THAR) and the dime-sized patch that could dominate a $15 billion market.
Interested investors should share the link with their broker or financial advisor.
It’s likely that you’ll both agree that Tharimmune (Nasdaq: THAR) could soon join the legion of sensational biotech startups.
 P.S. Still want more information on Tharimmune, Inc. (THAR)?
P.S. Still want more information on Tharimmune, Inc. (THAR)?
Sign up below for Tharimmune’s Investor Presentation, and breaking news about this and other interesting companies, completely free. Sign up below to learn about this investment opportunity.
By signing up above, Wall Street Fundamentals will send the latest investor report to the email provided. You'll also receive special reports completely free. You can unsubscribe at any time. View our Privacy Policy here.
IMPORTANT NOTICE AND DISCLAIMER
[1]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[2]https://www.researchgate.net/publication/49720316_A_new_transmucosal_drug_delivery_system_for_patients_with_breakthrough_cancer_pain_The_fentanyl_effervescent_buccal_tablet
[3]
https://www.clexio.com/pipeline/cle-400-pruritus/
[4]
https://www.liver.theclinics.com/article/S1089-3261(22)00047-2/fulltext
[5]
https://bmjopengastro.bmj.com/content/9/1/e000857
[6]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[7]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[8]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[9]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[10]
https://www.labce.com/spg1094059_first_pass_hepatic_metabolism.aspx
[11]
https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone-and-nalmefene
[12]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[13]
https://ir.tharimmune.com/wp-content/uploads/2024/05/Tharimmune-CORP-DECK-5.31.24v2.pdf
[14]
https://resource-allocation.biomedcentral.com/articles/10.1186/s12962-020-00223-x
[15a]
https://ir.tharimmune.com/wp-content/uploads/2024/01/THAR-Corp-Presentation-January-2024.pdf
[15b]
https://ir.tharimmune.com/wp-content/uploads/2024/01/THAR-Corp-Presentation-January-2024.pdf
[16]
https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity